

A battery is a device that converts chemical energy contained within its active materials directly into electric energy by means of an electrochemical oxidation-reduction (redox) reaction. This type of reaction involves the transfer of electrons from one material to another via an electric circuit.
A battery is a device that converts chemical energy contained within its active materials directly into electric energy by means of an electrochemical oxidation-reduction (redox) reaction. This type of reaction involves the transfer of electrons from one material to another via an electric circuit.
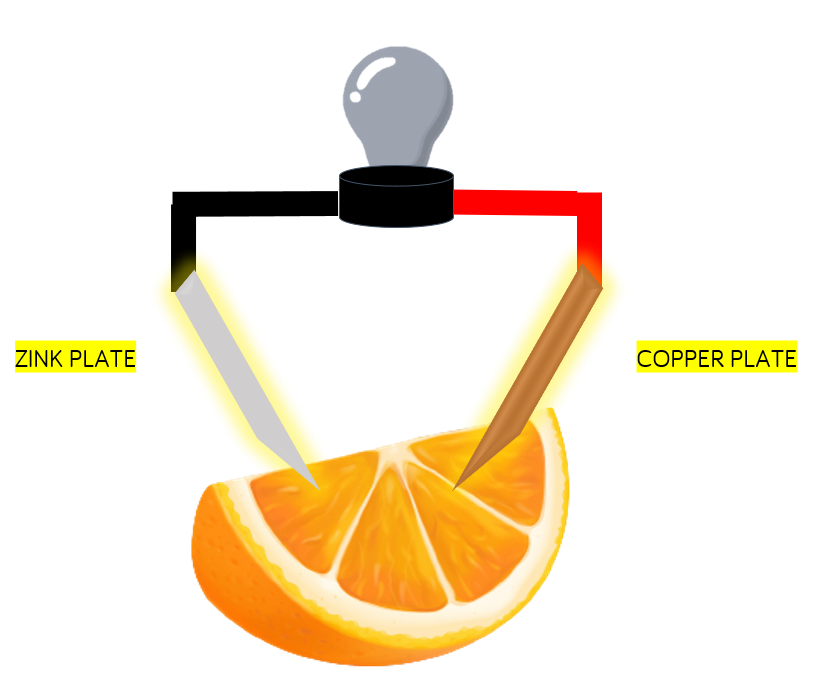
Electrolytic cell is the cell in which electrolysis of an electrolyte is done i.e., electrical energy is used to produce chemical energy. Whereas, Electrolysis is used to break chemical bonds via a catalyst in a solution. This converts some electrical energy into potential chemical energy. Electrical energy does not directly convert to chemical energy. They interact through several processes, electrolysis being one of the most common.